Understanding Oxidative Stress: The Role of Electron Transfer
Written on
Chapter 1: Introduction to Oxidative Stress
Oxidative stress refers to an electrochemical phenomenon that revolves around the improper rate of electron movement. What consequences arise when this electron transfer is not properly controlled?

Photo by Max Bender on Unsplash
Electrons play a crucial role in conducting electric currents. For instance, when we turn on a light switch, we are completing a circuit that allows electrons to flow and convert electrical energy into light, illuminating our homes.
In chemical interactions, however, the movement of electrons from one substance to another can modify their physical and chemical characteristics. This is where oxidation and reduction come into play. When one substance, Chemical A, extracts electrons from another, Chemical B, we classify Chemical B as being oxidized (losing electrons), while Chemical A acts as a pro-oxidizing agent that becomes reduced.
For example, steel, composed of iron, is susceptible to rusting, which occurs when iron interacts with oxygen in the air. Oxygen, being electron-deficient, easily removes excess electrons from iron, effectively oxidizing it into iron (III) oxide, commonly known as rust. Here, iron loses electrons and gets oxidized, while oxygen gains them and is reduced. Iron (III) oxide is stable and does not typically undergo further oxidation or reduction under standard conditions.
However, if iron becomes chemically unstable during oxidation, it may seek to reclaim lost electrons by breaking down other stable compounds. This can lead to the formation of reactive oxygen species (ROS), which then strive to oxidize other substances to regain electron stability, initiating further ROS reactions and generating free radicals.
Section 1.1: Understanding Redox Potential
A stable version of Chemical B can reduce the risk of ROS propagation. Different pairings of A and B yield varied reduction-oxidation (redox) potentials, as illustrated in the table below:
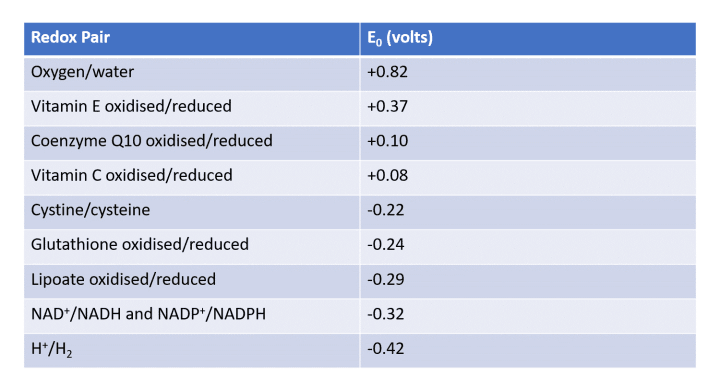
Table 1: Redox pairs in the human body (sourced from a journal article)
The table reveals numerous redox pairs related to various nutrients found in our diet or bodies. The redox potential (E0) of a pair indicates its oxidizing ability. A higher positive value suggests a greater likelihood of oxidation, while a more negative value indicates a tendency to be reduced.
For instance, Vitamin E has a redox potential of +0.37 V, while Vitamin C stands at +0.08 V. This implies that the reduced form of Vitamin C can effectively oxidize Vitamin E, causing Vitamin E to revert to its reduced state. The overall redox potential calculation of +0.37 - (+0.08) = +0.29 V confirms the spontaneity of this reaction. Reduced Vitamin C can regenerate oxidized Vitamin E, which acts as an antioxidant in the body, leading to its oxidation by pro-oxidant ROS.
Glutathione is recognized as the "master antioxidant" since our cells can produce and regenerate it through the glutathione reductase enzyme. Its position in the table illustrates its capability to restore depleted dietary antioxidants like Vitamin C and E.
Section 1.2: The Implications of Dysregulated Electron Transfer
Returning to the matter of regulating electron transfer, the redox reactions in our bodies are typically well-managed. While mitochondria generate a significant amount of ROS, the antioxidant glutathione, produced by the nuclear factor erythroid 2-related factor 2 (nrf2) pathway, regulates ROS activity.
If ROS production surpasses what glutathione can neutralize, our dietary antioxidants, such as Vitamins C and E, serve as a secondary defense. However, if these antioxidants cannot counterbalance the excess ROS, an imbalance occurs, leading to oxidative stress. This stress can alter the structure and function of proteins, lipids, and DNA.
Although oxidative stress may seem like a chemical issue, it signals deeper biological alterations. Just as psychological stress can impair decision-making, oxidative stress can hinder cellular function. For instance, consider the regulation of blood pressure.
Chapter 2: Blood Pressure Regulation and Oxidative Stress
Endothelial cells lining our blood vessels produce an enzyme called endothelial nitric oxide synthase (eNOS). This enzyme works with a cofactor, tetrahydrobiopterin (BH4), to generate nitric oxide (NO), which signals blood vessels to widen (vasodilation).
During oxidative stress, several outcomes may occur:
- NO may transform into peroxynitrite radicals, losing its ability to facilitate vasodilation.
- BH4 may oxidize into dihydrobiopterin (BH2), resulting in eNOS decoupling and a reduced capacity to produce NO.
Consequently, endothelial cells struggle to function optimally under oxidative stress. With impaired vasodilation, blood vessels constrict more easily, forcing the heart to exert greater pressure to circulate blood. This perpetual constriction can lead to hypertension, indicating that hypertension is, indeed, a cellular dysfunction.
In summary, while mitochondrial energy production generates ROS, our bodies have internal systems, like glutathione, to counteract these reactive species. If ROS production exceeds neutralization capabilities, oxidative stress emerges, jeopardizing our biochemical pathways. This imbalance disrupts our body's homeostasis.
Exploring the Goldilocks Principle for Health
Anything unregulated can spiral out of control, and we certainly want to avoid that.
To support our body's antioxidant production, we can upregulate the nrf2 pathway, enhancing our internal defenses against oxidative stress.
Antioxidant Protection Begins Within
It’s crucial to recognize that our cells produce their own antioxidants.
Ultimately, we need to remain aware that oxidative stress can arise from our actions, often unbeknownst to us. Let’s strive to maintain better health and well-being.
The video "The Electron Transport Chain Explained (Aerobic Respiration)" provides insights into how electron transfer processes influence cellular respiration and oxidative stress.